JENKINTOWN, Pa., May 2, 2024 /PRNewswire/ — SFA Therapeutics, Inc., a clinical-stage biopharmaceutical company developing oral small-molecule biosynthetic compounds for the treatment of inflammatory diseases, today announced the appointment of Stefan C. Weiss, MD, MBA, as Chief Medical Officer.
Dr. Weiss is a physician-scientist and entrepreneur with 25 years of experience in the healthcare and life sciences industries. Dr. Weiss leverages his clinical expertise and business acumen to lead teams through patient care and complex business and strategic implementations. A leader in the field of dermatology, Dr. Weiss is a practicing dermatologist who has secured multiple new drug approvals for the treatment of inflammatory skin diseases and has been selected by his peers as one of the nation’s top doctors. Dr. Weiss has authored more than 100 peer-reviewed abstracts, posters, and manuscripts.
“Dr. Weiss brings decades of multi-faceted clinical and industry experience in both dermatology and immunology,” commented Ira Spector, Ph.D., CEO of SFA Therapeutics. “His expertise in dermatologic therapeutic development will be indispensable for the company as we advance SFA-002 in clinical trials for the treatment of psoriasis. Dr. Weiss will also advise us on the best course of action for leveraging our platform to develop additional therapeutic candidates for other indications.”
“I am honored to join the SFA Therapeutics team to help shape the clinical pathway forward for SFA-002 and other candidates that target inflammatory disease. As a practicing dermatologist, I have witnessed the immense need for novel therapeutics with greater efficacy and fewer side effects than the currently available treatments for chronic inflammatory skin conditions,” stated Dr. Weiss, Chief Medical Officer of SFA Therapeutics. “I am confident in SFA Therapeutics’ novel approach which acts on multiple therapeutic pathways by using endogenous biosynthetic compounds. SFA Therapeutics is well-positioned to provide potentially safer and more efficacious treatments for chronic inflammatory diseases like psoriasis.”
Most recently, Dr. Weiss was the Managing Director of Dermatology at OM1, a company focused on health care data analytics and artificial intelligence. In this position, he was responsible for deepening OM1’s clinical focus and expertise around Immunology. He provided guidance on the scientific standards and clinical relevance of real-world data offerings across immunologic diseases. In addition, he oversaw the development of prospective studies to collect real-world evidence, including the ClinPro Derm™ Protocol.
Dr. Weiss received his undergraduate degree with honors from Yale University, his MD and MSc in clinical research from the Duke University School of Medicine, and his MBA from the Kellogg School of Management at Northwestern University. He completed a residency in Dermatology at Stanford University and a Fellowship in Bioethics at the National Institute of Health.
About SFA Therapeutics
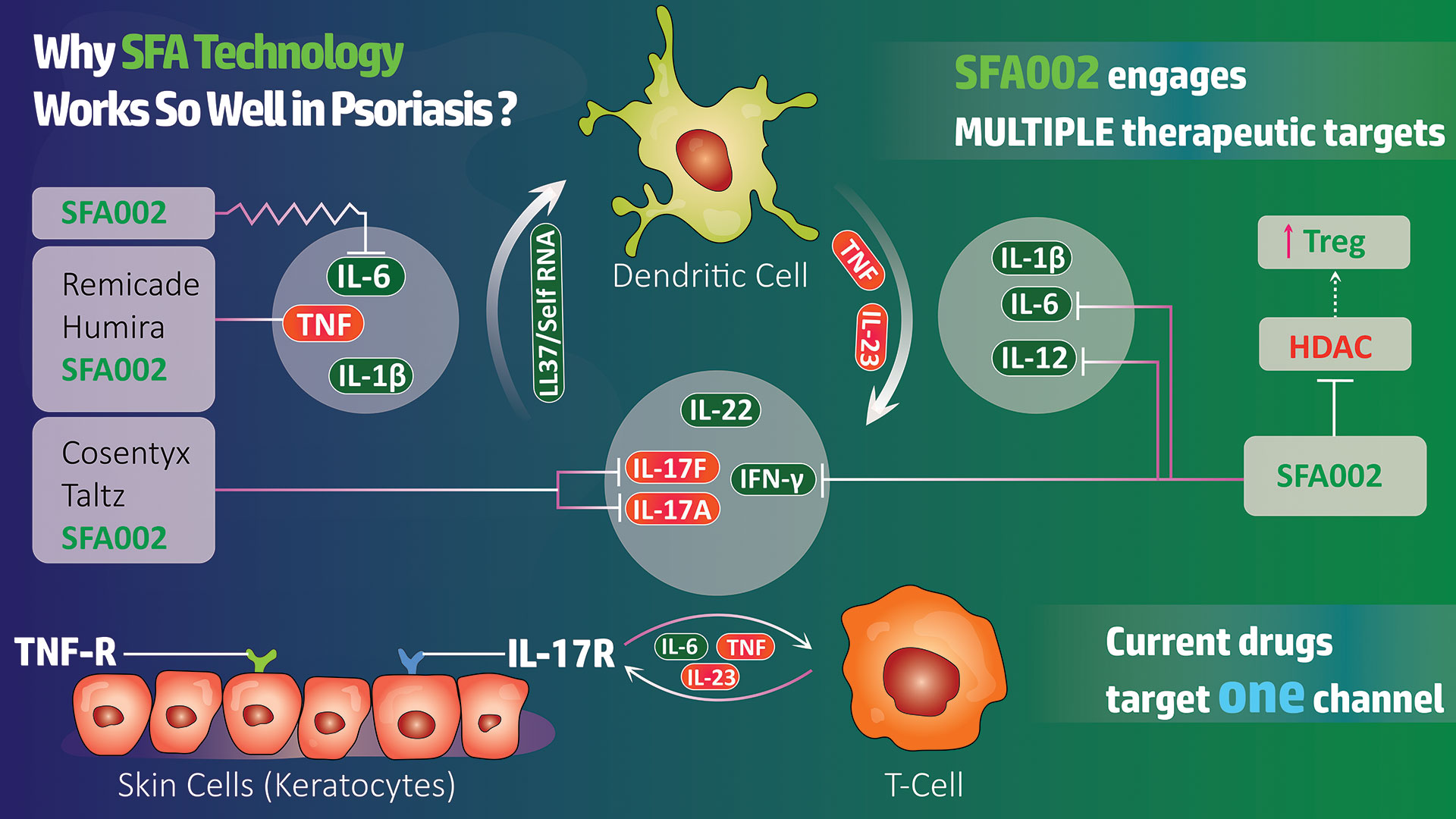
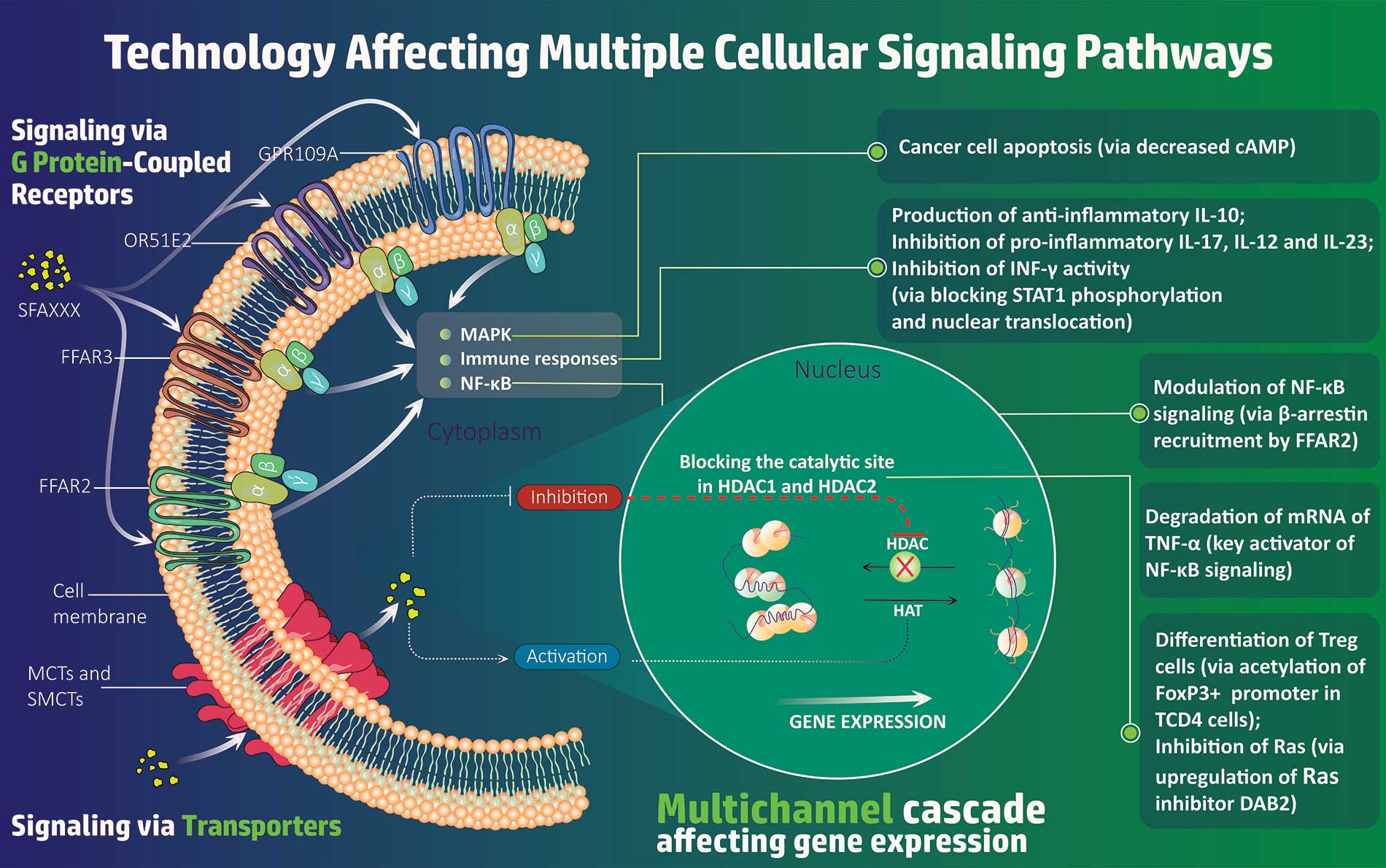
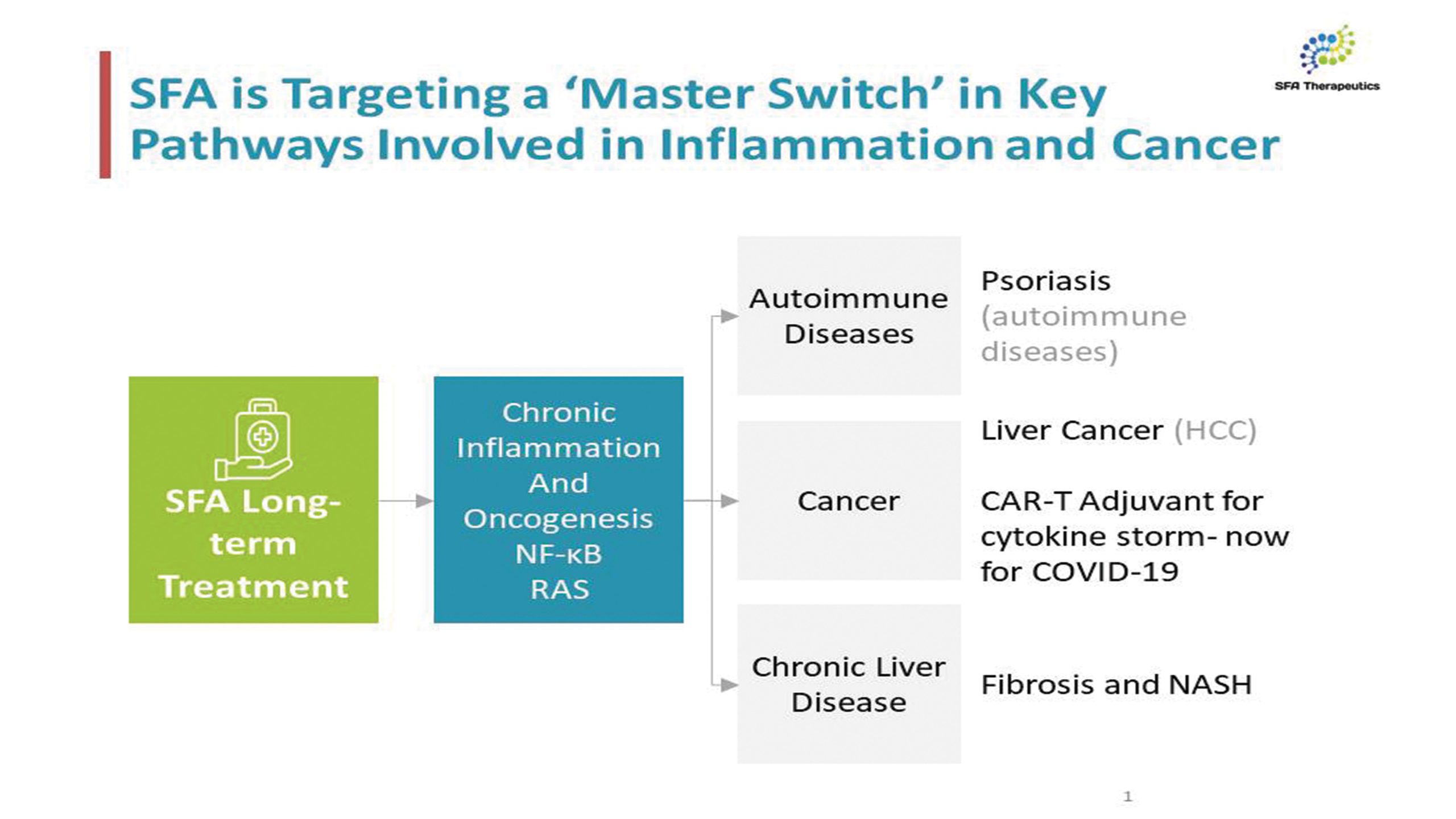
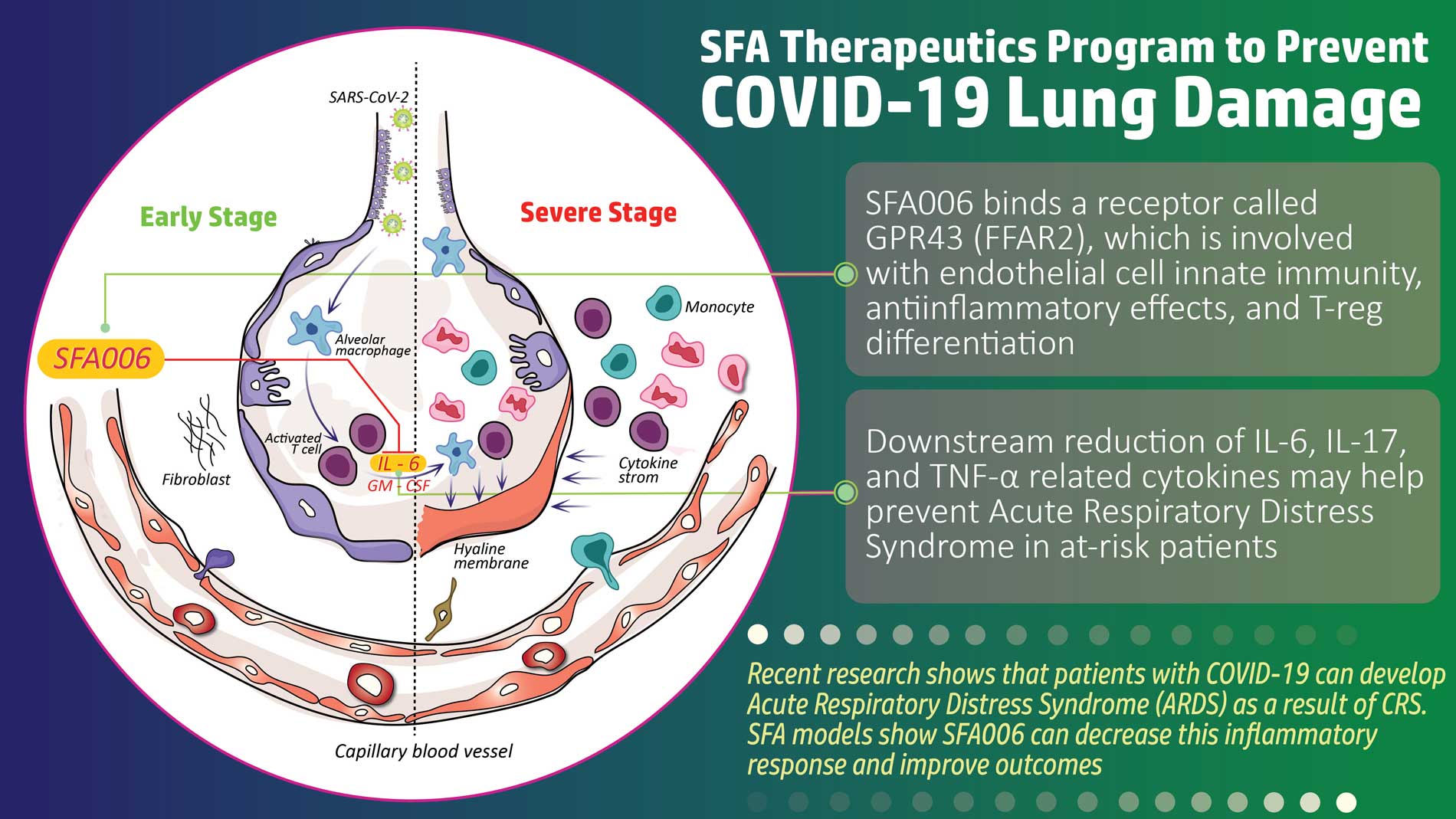
SFA Therapeutics, Inc. is a clinical-stage biopharmaceutical company developing oral small-molecule biosynthetic compounds for the treatment of inflammatory diseases. Based on breakthrough research licensed from Temple University, SFA Therapeutics’ platform has the potential to develop safer and more efficacious treatments for a number of chronic inflammatory diseases by uniquely tailoring the effects of patented formulations with target-specific adjuvants. Its lead asset, SFA-002, is approaching Phase 2 clinical trials and has shown promising Phase 1a and Phase 1b results for the treatment of psoriasis. SFA Therapeutics has also received clearance of its Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) to investigate SFA-001N in patients with metabolic dysfunction-associated steatohepatitis (MASH), also known as non-alcoholic steatohepatitis (NASH), with or without fibrosis. SFA Therapeutics has an Orphan Disease Designation from the FDA for SFA-001 in the treatment of hepatocellular carcinoma, the most prevalent form of liver cancer.
SFA Therapeutics is headquartered in Jenkintown, Pennsylvania. Please visit www.sfatherapeutics.com to learn more.
Company Contact
Ira Spector, Ph.D.
SFA Therapeutics, Inc.
+1 267-584-1080
Media Contacts
Tony Russo, Ph.D.
Russo Partners, LLC
Tony@RussoPR.com
Maddie Stabinski
Russo Partners, LLC
Madeline@RussoPR.com
SOURCE SFA Therapeutics, Inc.