JENKINTOWN, PA, UNITED STATES, April 8, 2021 /EINPresswire.com/ — SFA Therapeutics, Inc., a clinical-stage biotechnology company, is pleased to announced the start of our first clinical trial for the treatment of “the appearance of the structure of skin with plaques due to psoriasis.” SFA plans to expand the evaluation of one of its six microbiome-derived drugs, SFA002, in this new trial.
This 30-subject open-label clinical trial in patients with mild-to-moderate psoriasis will enroll 30 subjects into two cohorts with 15 subjects each. In the first group, 15 patients will be treated with an initial formulation of SFA002, and in the second group an additional 15 subjects will be treated with a second formulation. Treatment duration will be three months, after which patients will be observed for an additional three months, and then statistical analyses will be conducted on the outcomes.
The Need for an Oral Treatment of Mild to Moderate Psoriasis Remains Acute
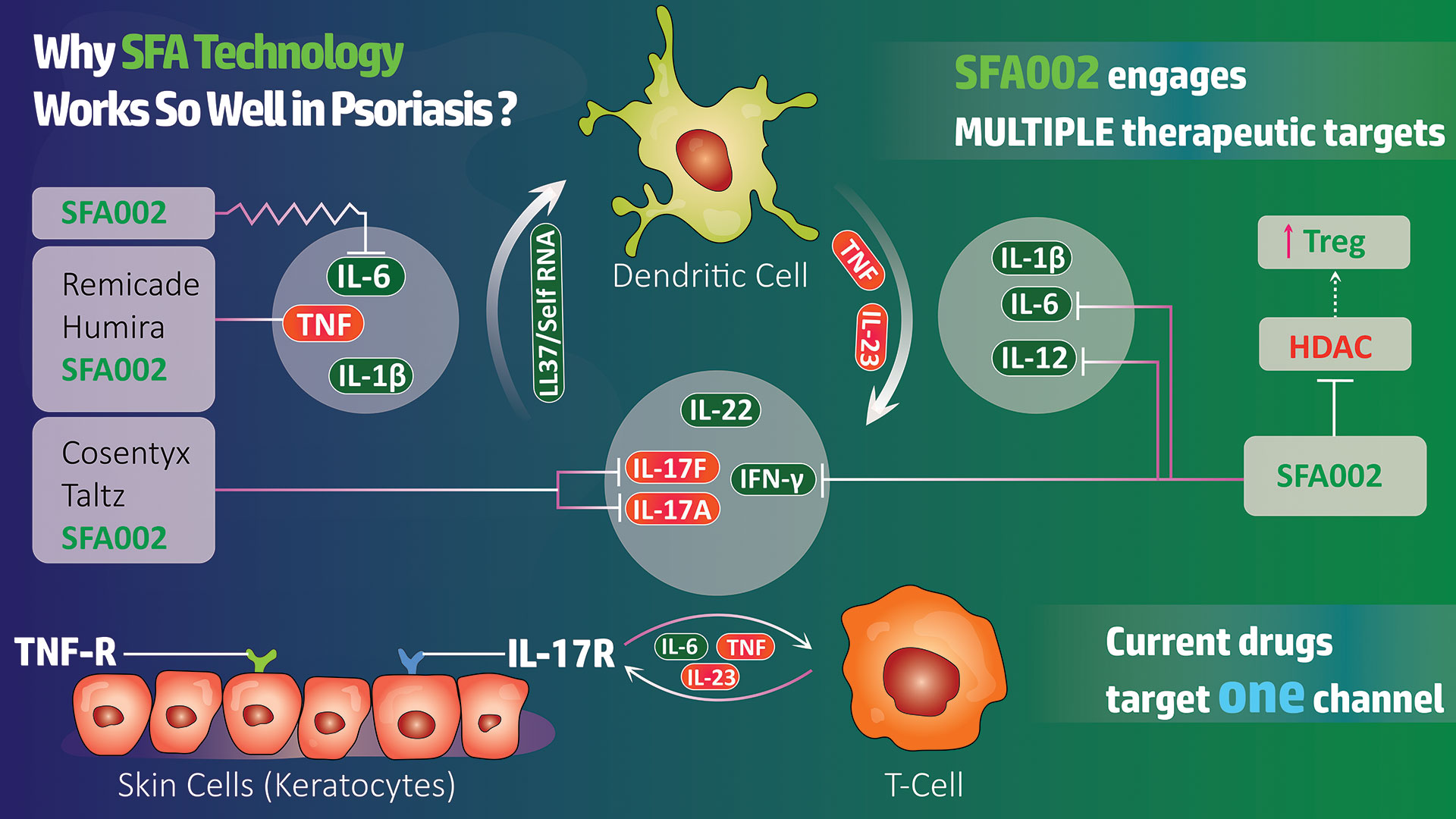
“Significant clearing of psoriatic plaques has already been demonstrated with SFA002 in a small study” commented SFA CEO Dr. Ira Spector, “and, because we know that this patient group has lacked an effective and safe oral treatment option, we are going into this larger IND trial with considerable optimism.”
Study recruitment is now open for enrollment. Following the trial’s completion, SFA expects to finalize the SFA002 formulation and proceed into a randomized controlled clinical trial.
The study is being conducted under the supervision and leadership of Dr. Sylvia, Hsu, Professor and Chair of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, PA.
For potential patient enrollment, please contact Dr. Sarmina Hassan at sarmina.hassan@tuhs.temple.edu or 215-707-7278.
About SFA Therapeutics
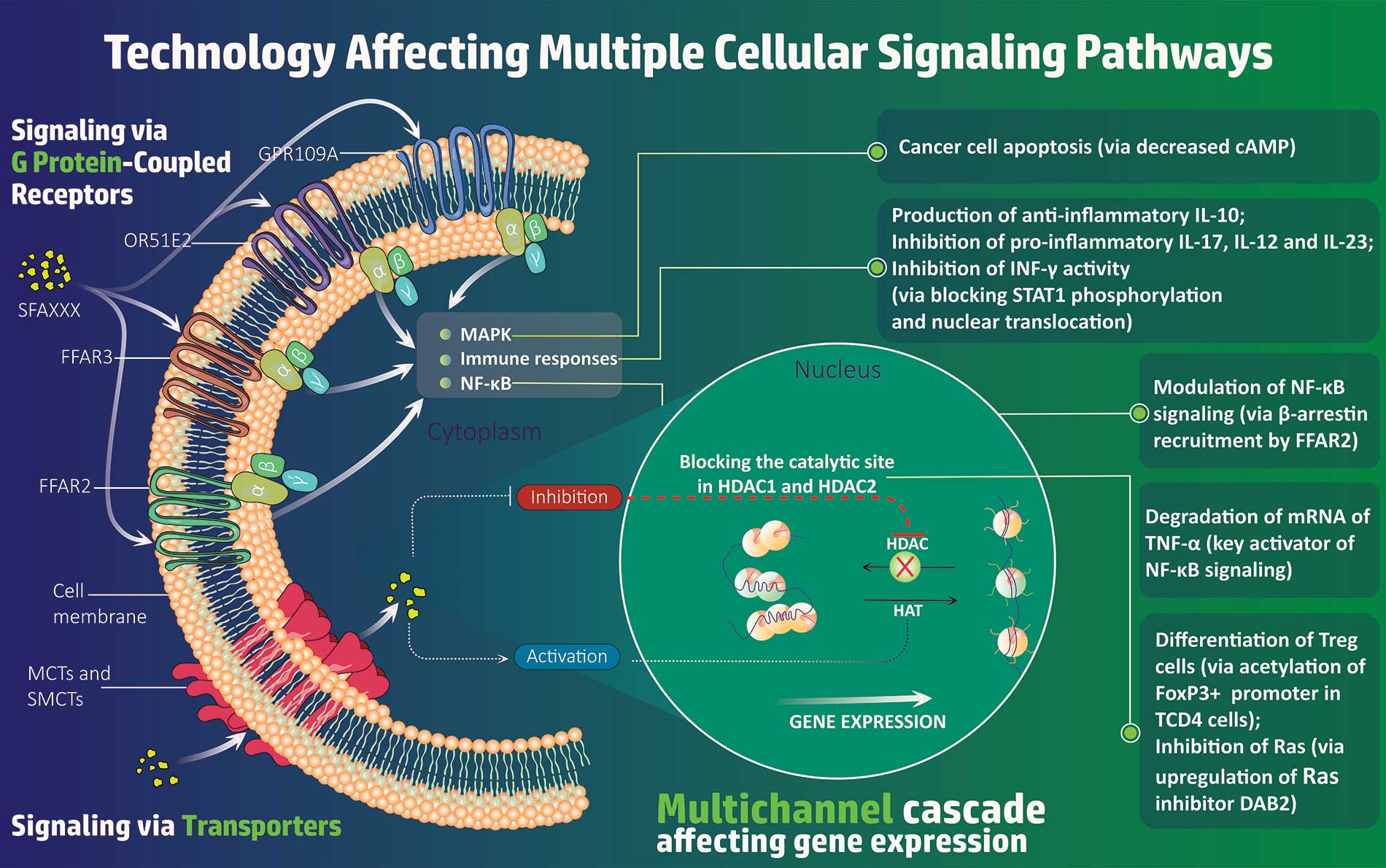
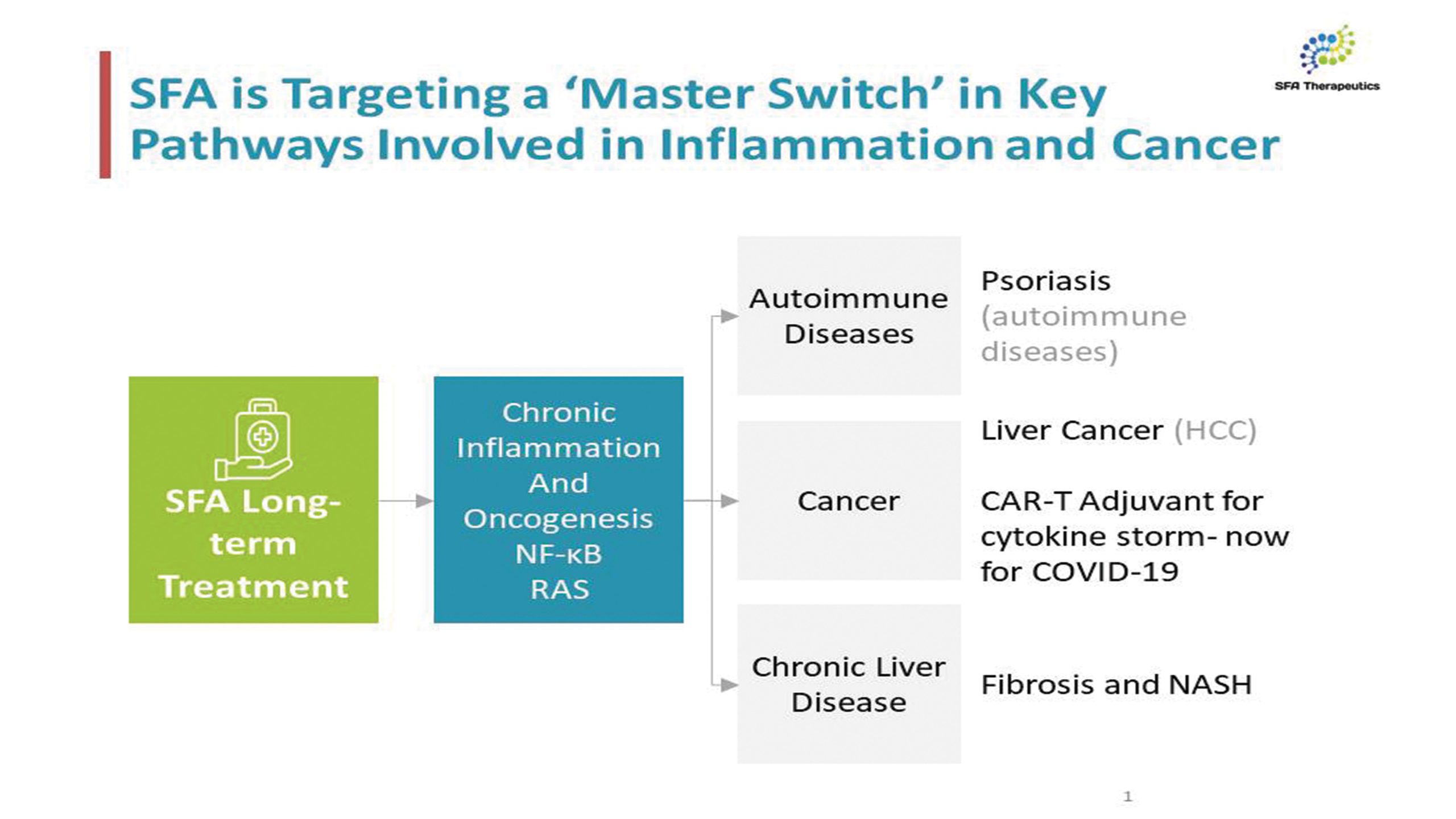
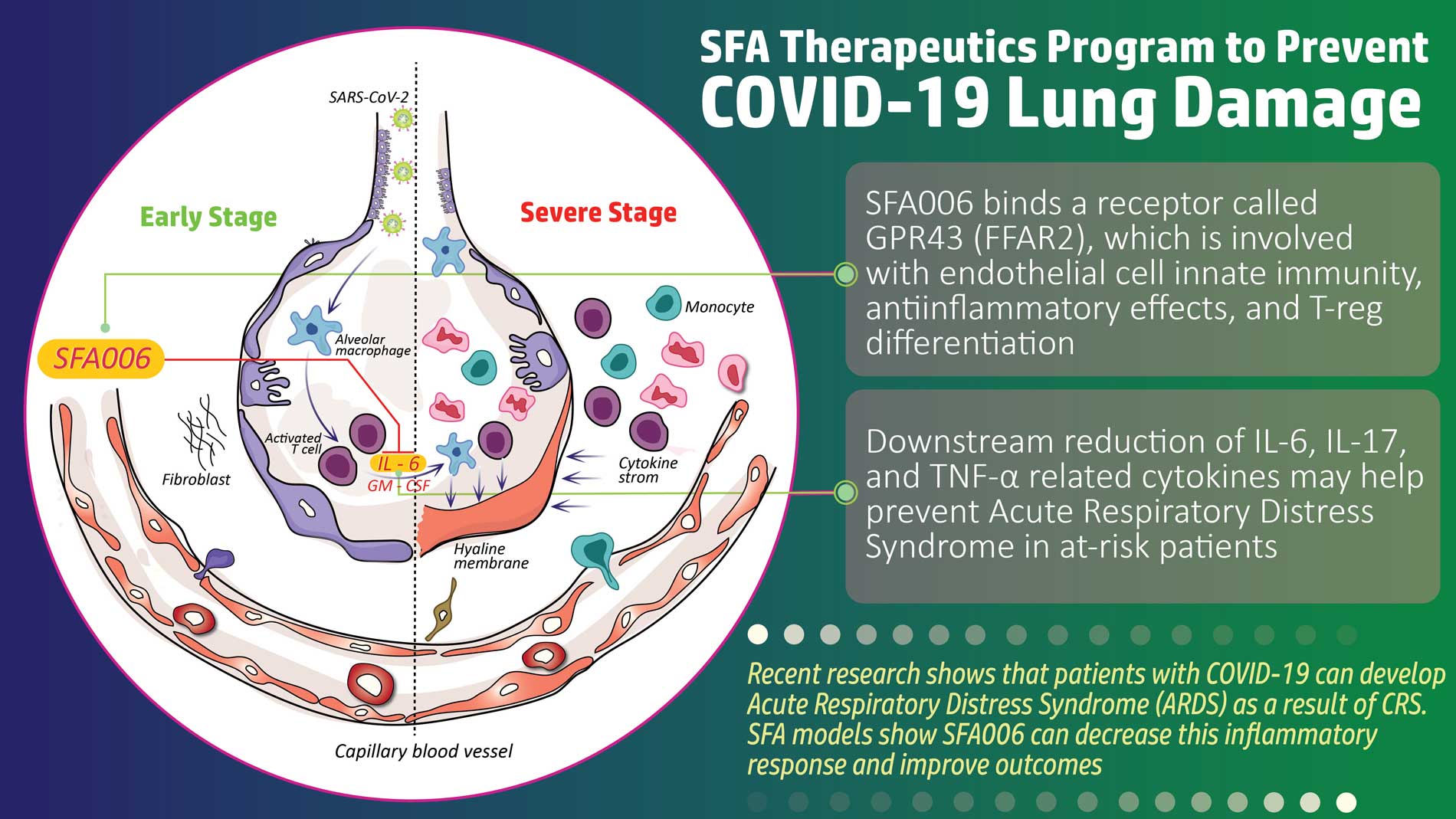
SFA Therapeutics, Inc., a spinout of Temple University, is a bio-pharmaceutical company focused on new human-microbiome-based advancements in the treatment of inflammatory diseases, targeting NF-kB and RAS. SFA is focused on proprietary drugs to treat chronic inflammation, which been implicated in a wide range of diseases, including Hepatocellular Carcinoma (liver cancer), Psoriatic Arthritis, Rheumatoid Arthritis, Lupus (SLE), Inflammatory Bowel Disease (IBD), Crohn’s Disease, and Chronic Liver Disease. SFA currently has six drugs under development, and is targeting psoriasis, Hepatitis B and Hepatocellular Carcinoma (liver cancer) with their lead assets.
SFA’s drug development platform has been exclusively licensed from Temple University to SFA Therapeutics in Jenkintown, PA. Please visit https://sfatherapeutics.com/ to learn more.
For investment information, please contact Dr. Ira Spector, CEO, at iraspector@sfatherapeutics.com
Ira Spector
SFA Therapeutics, Inc.
+1 267-584-1080
email us here
Visit us on social media:
LinkedIn