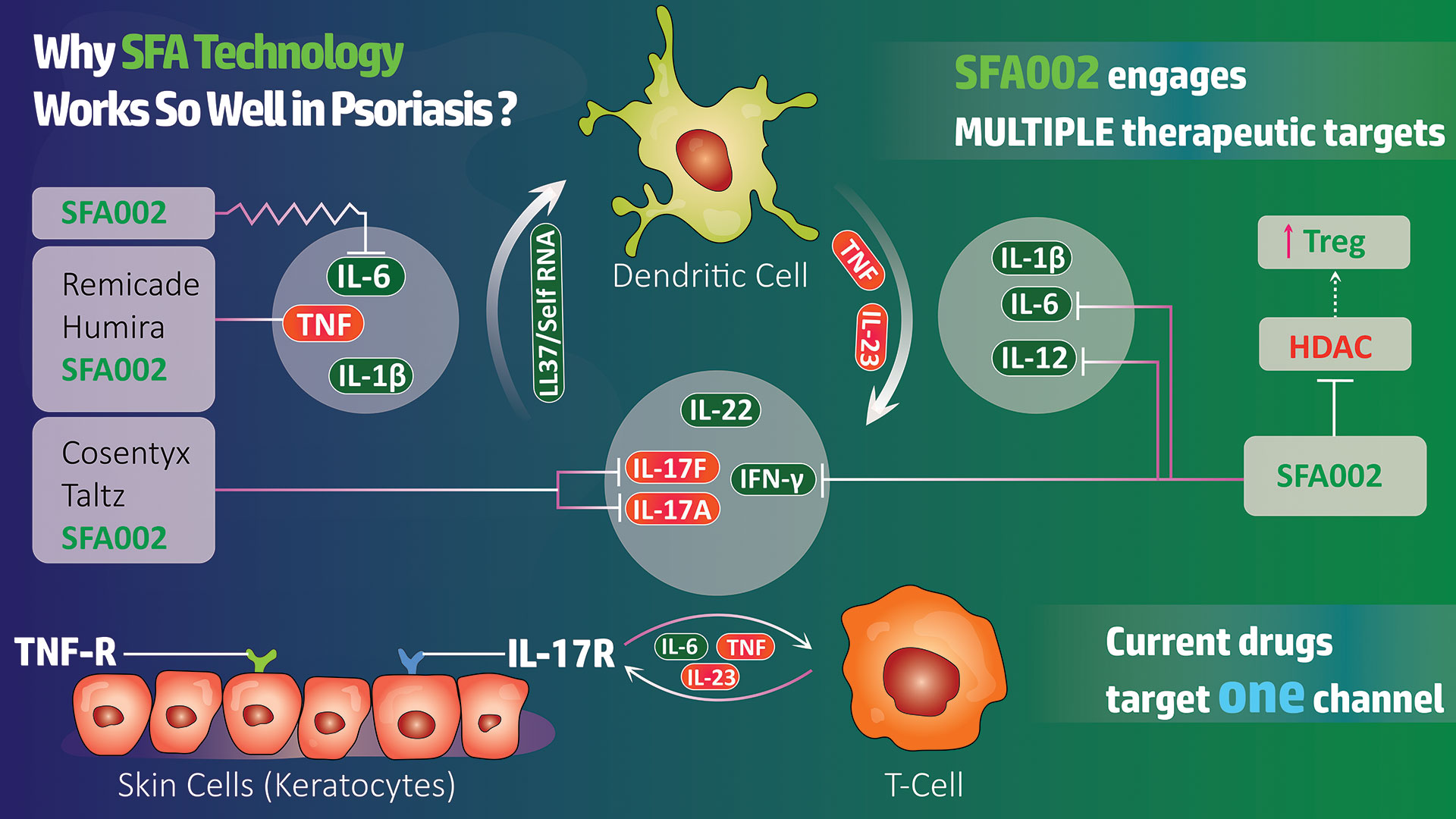
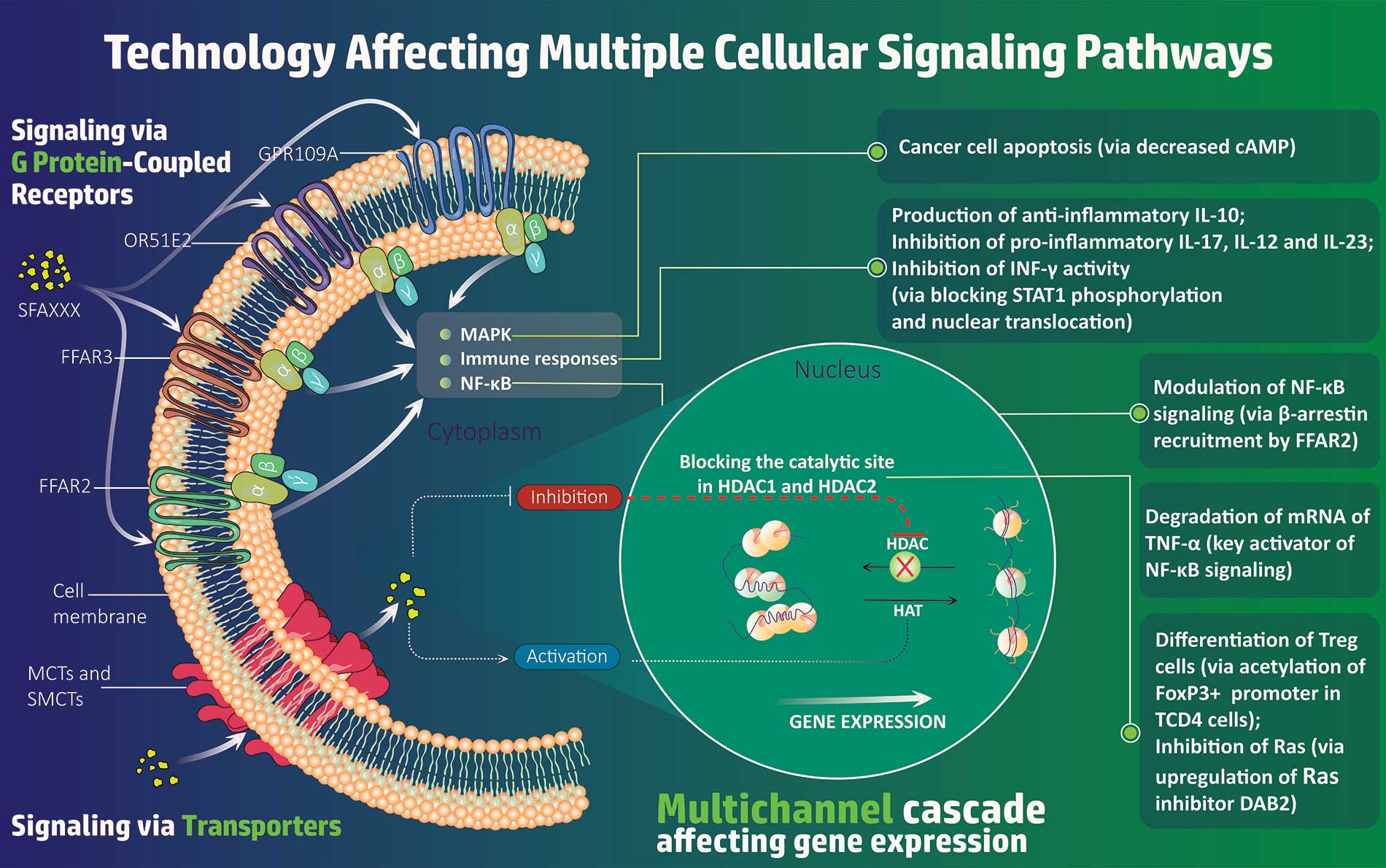
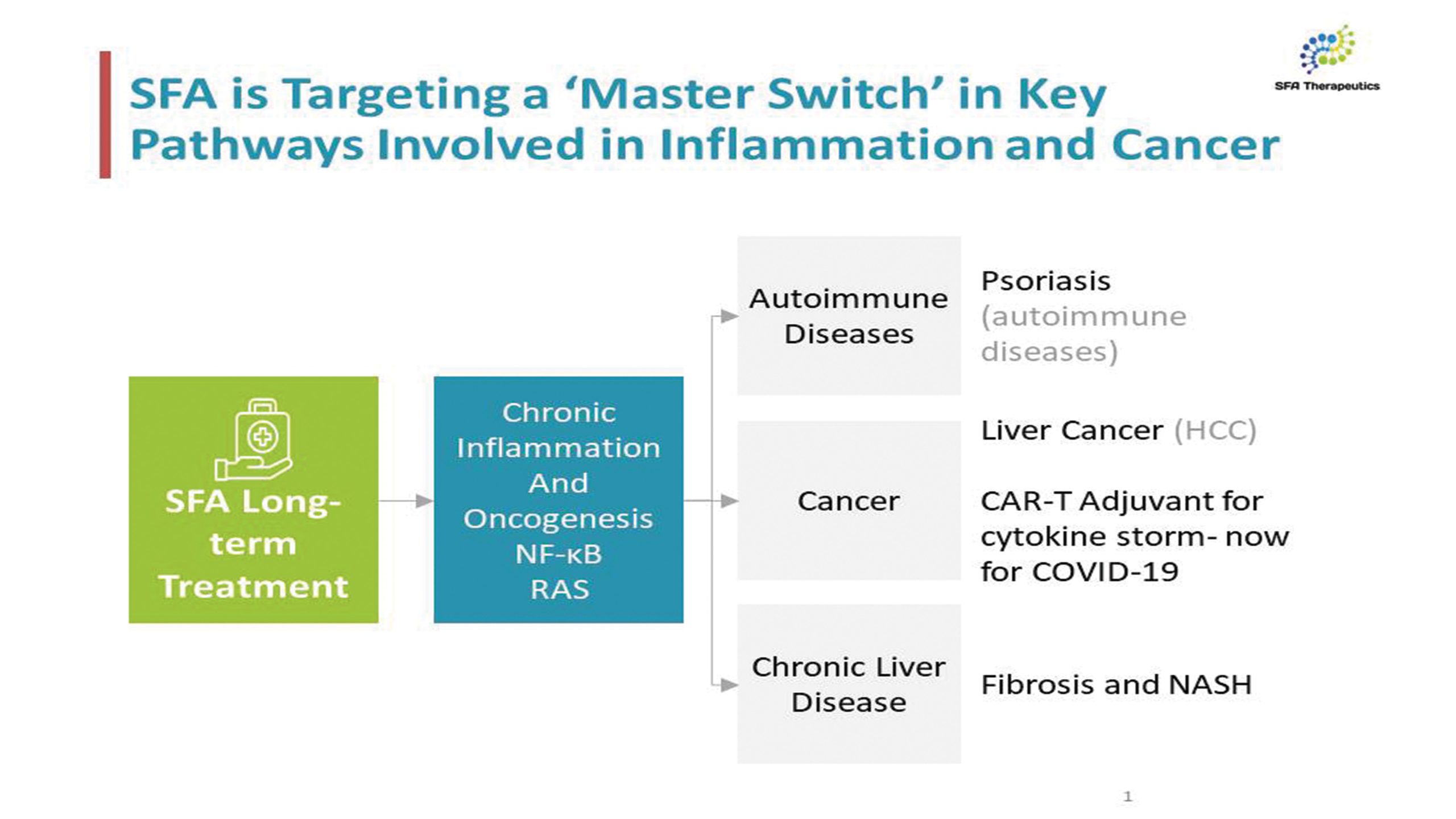
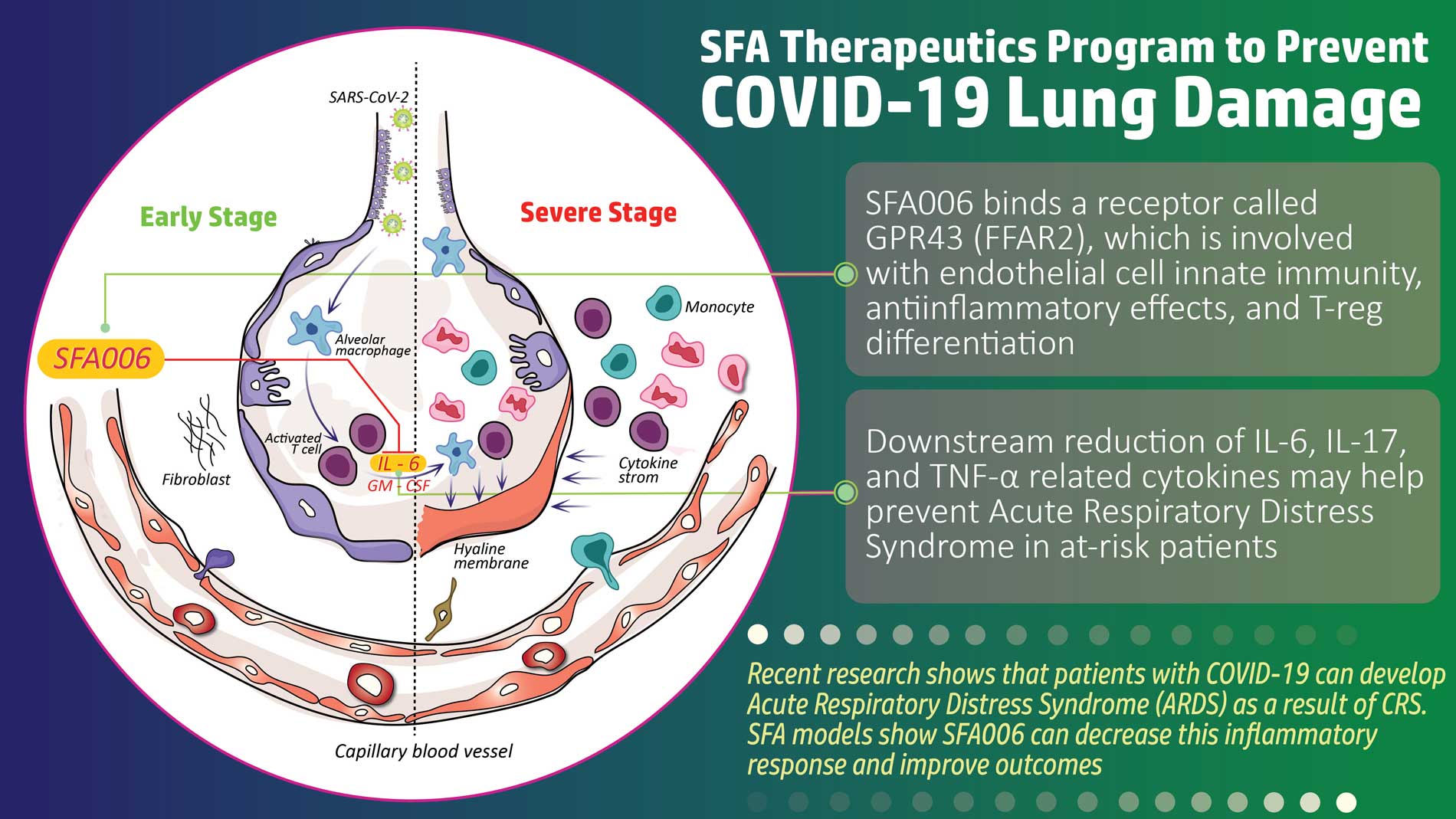
SFA Therapeutics will begin testing of SFA001 in a Phase 1 exploratory trial at Einstein Hospital in Philadelphia 1Q 2023
Dr Manisha Verma MD, MPH to act as principal investigator
SFA Therapeutics to test its novel anti-inflamaatory compound, SFA001 in NASH patients. SFA001 is a powerful microbiome derived orally active small molecule has been shown to greatly reduce chronic inflammation. Chronic inflammation leads to the activation of hepatic stellate cells that undergo trans-differentiation to become myofibroblasts, the main extra-cellular matrix producing cells in the liver; over time increased extra-cellular matrix production results in the formation of liver fibrosis. The study will explore the ability of SFA001 to reduce fibrosis in patients.